![Molecular Zinc Hydride Cations [ZnH]+: Synthesis, Structure, and CO2 Hydrosilylation Catalysis - Ritter - 2020 - Angewandte Chemie International Edition - Wiley Online Library Molecular Zinc Hydride Cations [ZnH]+: Synthesis, Structure, and CO2 Hydrosilylation Catalysis - Ritter - 2020 - Angewandte Chemie International Edition - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/8fb7eef0-0324-42ed-a2b2-135946ab80b4/anie202011480-toc-0001-m.png)
Molecular Zinc Hydride Cations [ZnH]+: Synthesis, Structure, and CO2 Hydrosilylation Catalysis - Ritter - 2020 - Angewandte Chemie International Edition - Wiley Online Library
43. Balance the following equation: Zn + (H+) —> (Zn+2) + H2 (Zinc reacts with hydrogen ion to give Zinc ion and Hydrogen gas.)
Consider the following reactions (unbalanced) Zn + hot conc. H2SO4 →G + R + X - Sarthaks eConnect | Largest Online Education Community

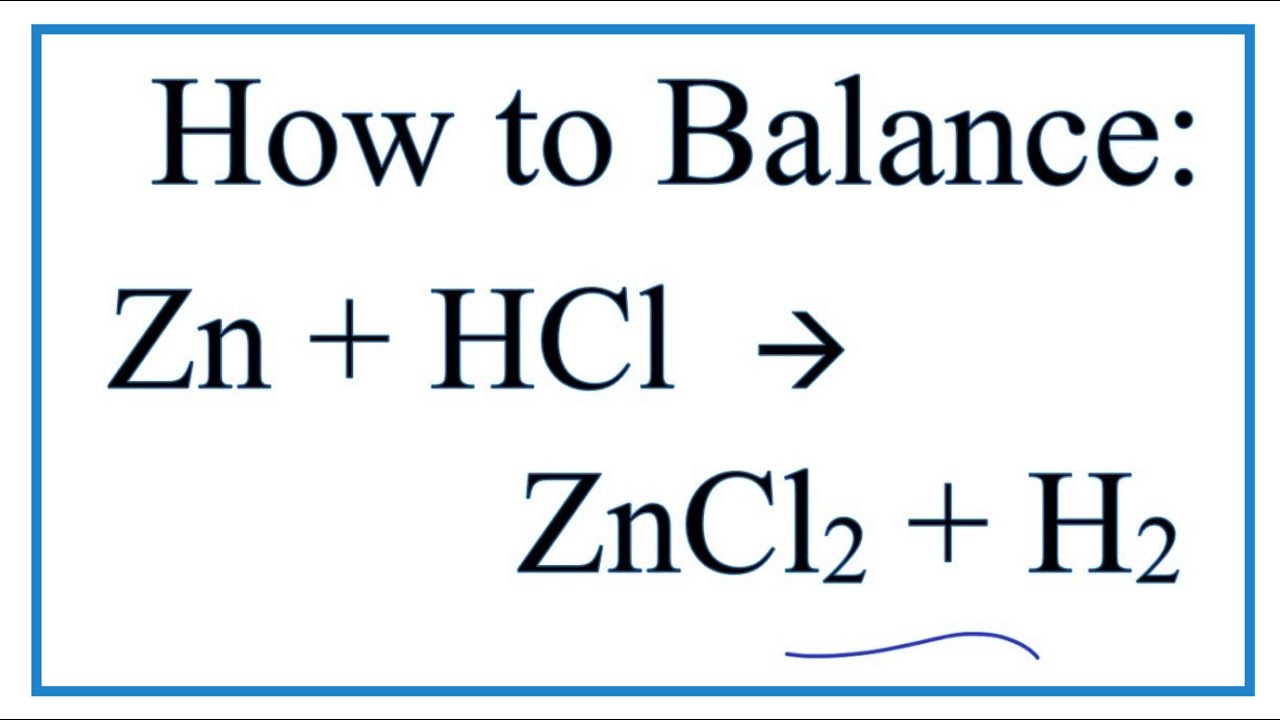
How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric Acid + Zinc) | How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric Acid + Zinc) Balancing equations could

Hydrosilane σ‐Adduct Intermediates in an Adaptive Zinc‐Catalyzed Cross‐dehydrocoupling of Si−H and O−H Bonds - Patnaik - 2021 - Chemistry – A European Journal - Wiley Online Library
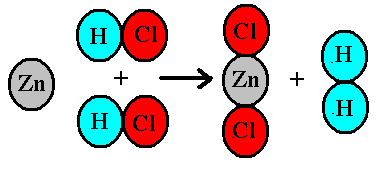
In the reaction Zn + 2HCl -> ZnCl_2 + H_2, how many moles of hydrogen will be formed when 4 moles of HCl are consumed? | Socratic

Trimetallic Cu–Ni–Zn/H-ZSM-5 Catalyst for the One-Pot Conversion of Levulinic Acid to High-Yield 1,4-Pentanediol under Mild Conditions in an Aqueous Medium | ACS Catalysis
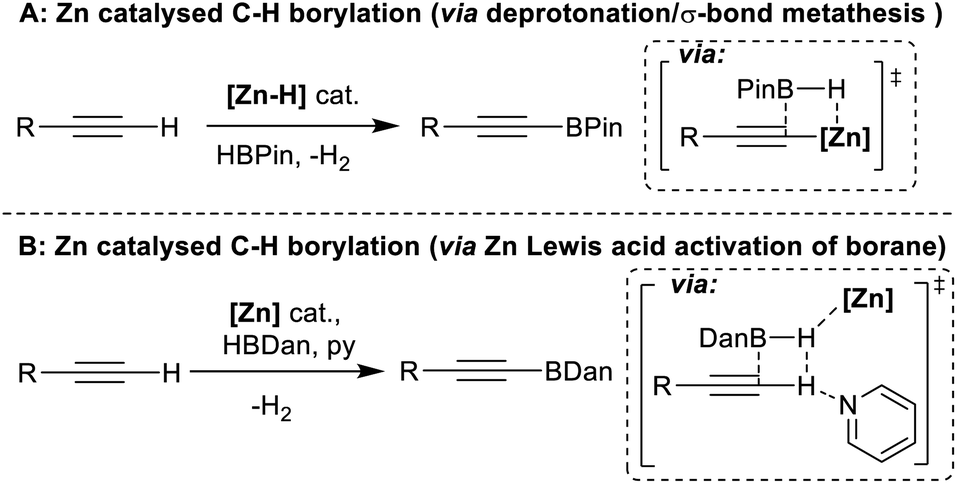
Zinc catalysed electrophilic C–H borylation of heteroarenes - Chemical Science (RSC Publishing) DOI:10.1039/D1SC01883C
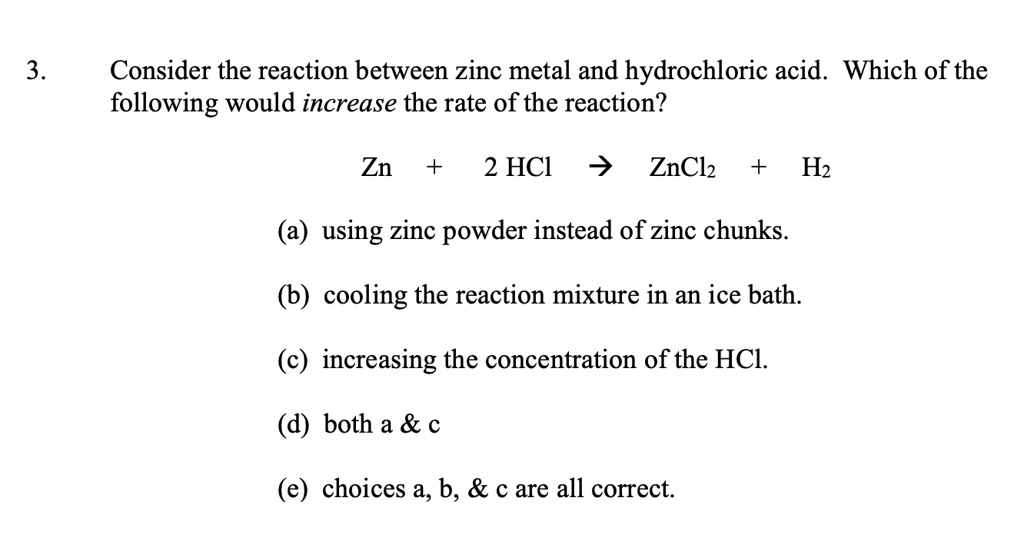
SOLVED: Consider the reaction between zinc metal and hydrochloric acid. Which of the following would increase the rate of the reaction? 3. ZnCl2 2. HCl Zn H2 using zinc powder instead of

SOLVED: In the reaction Zn + H+ —> Zn2+ + H2, Zn is oxidized and H is reduced. How many electrons would be produced and used in the balanced half-reactions? Complete the





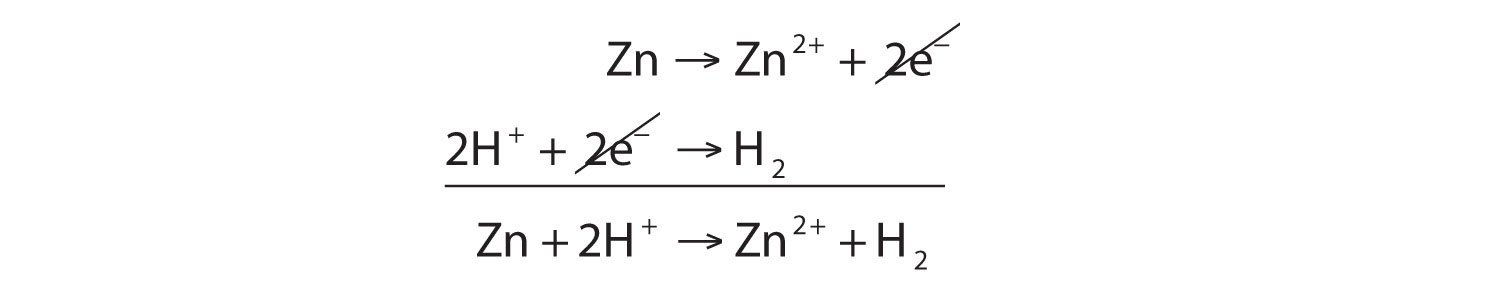



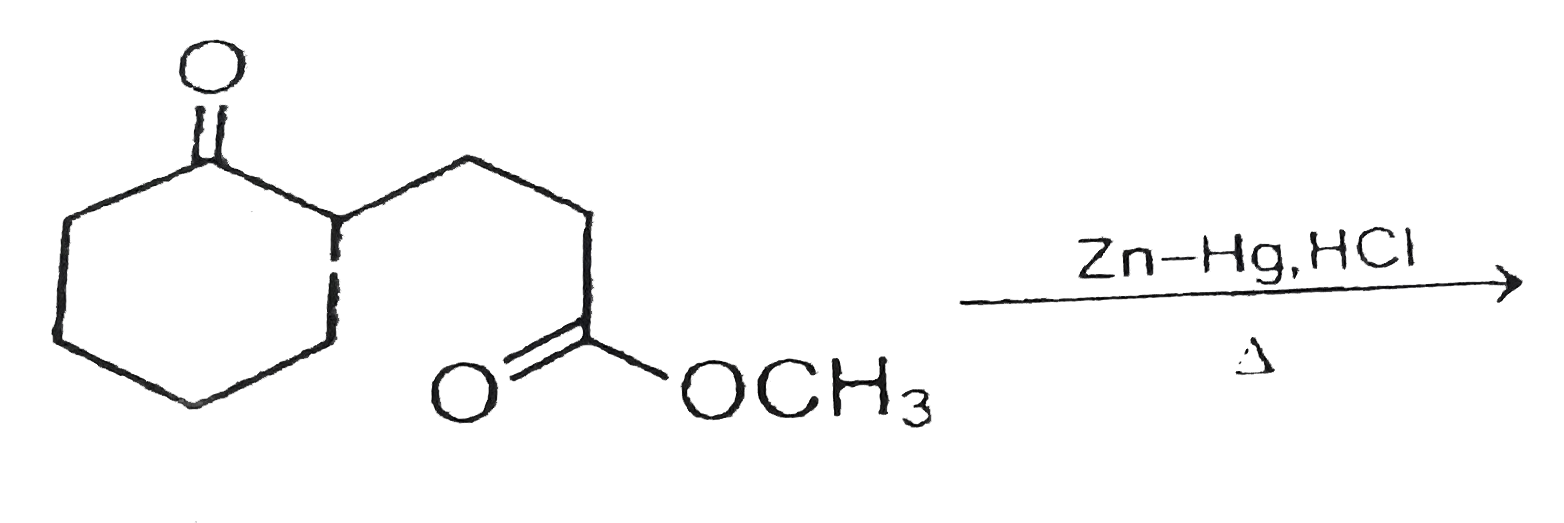
![In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is: In the reaction, C6H5COCH3 [Zn - Hg/conc. HCl][H]X . X is:](https://dwes9vv9u0550.cloudfront.net/images/7671796/5bdff3d8-c0c9-4fc8-89cd-f9235a0c1b30.jpg)